UDI Marking on Medical Devices
The US Food & Drug Administration’s (FDA) implementation of direct part marking of medical devices marches on.
This process of ushering in unique device identification (UDI) has become progressively binding since 2014.
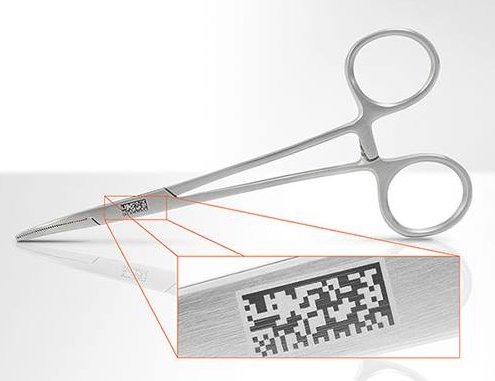
Set to complete in September 2020, the next deadline is set for September this year with Class II devices required to be marked with UDI on the actual device not just the packaging.
Why UDI?
There are four main reasons why the FDA is implementing UDI. These are:
- To provide better reporting.
- To reduce errors and enable faster error corrections (e.g. product recalls).
- To allow the devices to be linked to electronic health records.
- To make the supply chain more transparent.
UDI will also facilitate a public database where FDA UDI access is granted to everyone. Where America goes so does Europe. European legislation includes the MDR (Medical Device Regulation) defining implementation dates starting in 2023.
2018 Deadlines
As well as Class II device marking, Class I devices (non-deadly/mission critical medical supplies such as bandages) need to have UDI marked packaging.
Exemptions or exceptions from the class system need to be submitted to the UDI database.
Stand-alone Class I software (software that does not create major problems should it go wrong) also need to be UDI marked.
Bearing in mind the size of the US medical device market, it’s worth noting that these regulations are not optional!
Comprehensive Overview
The implementation of UDI marking on medical devices must comply with basic legislation and organisational regulations.
Our product partner, FOBA, has produced a Medical Industry White Paper providing a comprehensive overview of Unique Device Identification.
This covers:
- Composition and quality characteristics of a UDI mark.
- Different product risk categories.
- Time schedule of Implementation due dates.
- Information on GUDID, the central UDI database, issuing agencies, etc..
Click to download your free Medical Industry White Paper
To find out why laser marking is the most appropriate technology to fulfil your UDI requirements and for detailed information on the FOBA range of laser marking systems, please contact sales@tlm-laser.com