The A to Z of Unique Device Identification (UDI)
Taken from the article, Laser Marking - Creating High Quality UDIs for Traceability published in the July/August 2017 issue of Precision Manufacturing
UDI for Medical Devices
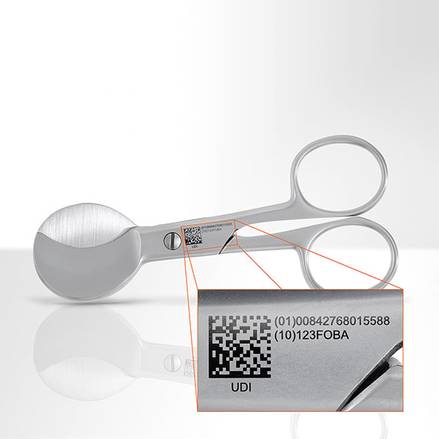
Since the Food & Drug Administration (FDA) announced the upcoming implementation of obligatory Unique Device Identification (UDI) marking for medical devices, manufacturers have been searching for feasible and economic marking solutions. The regulatory process – started in September 2014 and ending in 2020 – requires that all medical devices need to be equipped with a unique product number and Datamatrix code to ensure their traceability throughout the product life cycle.
Direct Part Marking
Direct part marking, in that context, is becoming increasingly important: if the product is reused and/or reprocessed before each use, the UDI marks need to be applied directly on the device. This requirement leads to the special significance of laser marking on plastic and metallic devices, which appears to be the only technology that is able to pass passivation processes while guaranteeing long-lasting marks that can resist high wear and abrasion as well as frequent sterilization cycles. In order to meet the labelling requirements, laser markings must be of high contrast, precise, long lasting, and able to fit into small spaces.
Patient Safety
The goal of the UDI system is to increase patient safety and to allow for sustainable traceability. The FDA directive applies to medical devices that are produced or imported and distributed in the U.S. Equivalent directives are being introduced internationally: the European Union has just released a resolution on UDIs within the Medical Device Regulation (MDR), becoming effective gradually from 2020
Identification
UDI codes consist of a Device Identifier (DI) and a Production Identifier (PI), the first of which identifies the manufacturer/labeller and the specific version or model of the device (also reference code). The DI is a globally unique product code which allows the clear identification of a device and must be entered in the central registry. The PI is optional and represents the variable portion of a UDI that identifies variables like the lot and batch numbers, serial numbers, expiration or manufacturing dates, etc..
Unique Codes
UDI codes are depicted graphically in an assembly of two forms: a human readable plain text of alpha-numeric characters and a machine-readable code. There are different forms of machine readable codes available: the GS1-128 linear bar code, the GS1 data matrix code from GS1, the HIBC linear bar code and the HIBC data matrix code, and the linear ISBT 128 and 2D code from ICCBBA
Depending on the accredited issuing agency, each product can receive its unique code in any of the above formats. The UDI system addresses all of the following aspects:
- More efficient product recalls.
- Improved counterfeiting protection.
- Simplification of data entry and accessibility with different systems.
- Security throughout the entire supply chain.
- Field Safety Corrective Action – FSCA.
- Better identification, documentation, and prevention of incidents.
- Prevent medical errors from occurring.
Additionally, the labelling system enables the simplification of logistics, ordering, and delivery processes.
For full details of FOBA’s laser marking machines including FOBA’s patented vision system (IMP), please contact sales@tlm-laser.com
To read this article in full, Laser Marking – Creating High Quality UDIs for Traceability, please visit www.mpma.com